The revolutionary new holder system enables for the use of standing inserts in all modules of the VITROCELL® 12 Series for 12-well sized inserts. As an additional advantage, the media […]
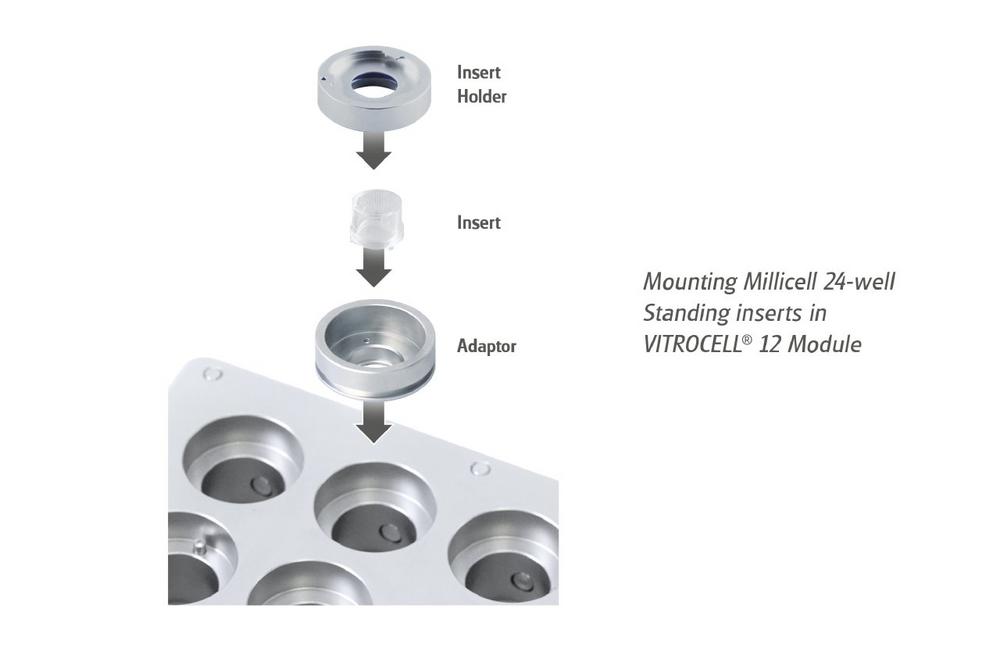

The revolutionary new holder system enables for the use of standing inserts in all modules of the VITROCELL® 12 Series for 12-well sized inserts. As an additional advantage, the media […]

The VITROCELL® RH/T-Controller system was designed to measure temperature and relative humidity in numerous applications at high precision. It can be fitted to humidification systems, aerosol ducts and climatic chambers. […]

The VITROCELL® Cloud α series for automated exposure of cell cultures has been enlarged and now comprises the Cloud Alpha 6, Cloud Alpha 12, Cloud Alpha 96 and Cloud Alpha MAX. […]

The VITROCELL® 12/12 module system has been designed to facilitate the exposure of mammalian cell cultures to airborne substances such as gases, complex mixtures, nanoparticles and fibers. It features a throughput […]
The VITROCELL® Cloud Alpha series is our newest innovation and presents a great leap forward in automated exposure of cell cultures. It combines reliable exposure of cell cultures from the […]

. Background Emissions of water pipe tobacco can now be tested by in vitro exposure in a reliable and user-friendly setup. The novel Shisha Testing Device can be combined with […]

The VITROCELL® Cloud Alpha 96 is our newest innovation and presents a great leap forward in automated exposure of cell cultures. It combines high throughput testing with ease of use. […]

New designs of electronic cigarettes such as ENDS (Electronic Nicotine Delivery Systems) products or HTP (Heated Tobacco Products) lead to a large variety of different shapes which make the insertion […]

. Background The assessment of toxicological effects of airborne particles to the human organism is of major importance in disease research. The presence of nano and ultrafine particles can be […]

In der Inhalationstherapie werden pharmazeutische Präparate als Aerosole auf Zellen des Atemtraktes im Nasen- oder Lungenbereich appliziert. Zur präklinischen Entwicklung neuer Arzneimittel werden dafür oft kommerziell erhältliche, physiologisch relevante in […]