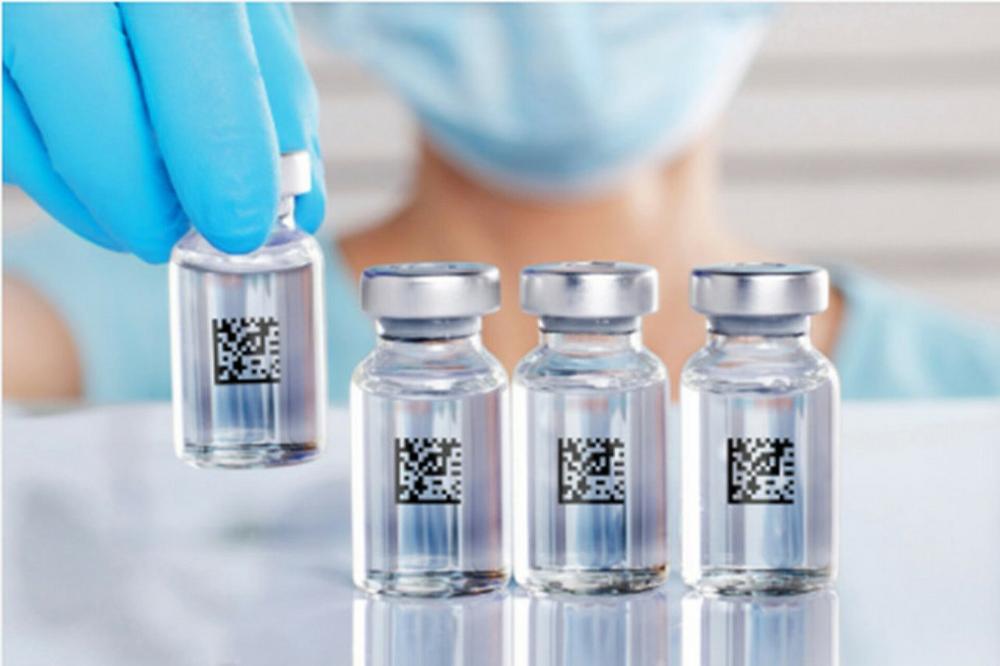
"Our vision includes transparency and traceability across the entire supply chain, from primary packaging to secondary packaging to filling and serialization of medicines to final use at the PoC," says Lea Amsted, digital strategy lead manager for the project.
Increase patient safety
Clearly marked primary packaging helps minimize the risk of costly and dangerous mix-ups when filling medicines. This factor is becoming increasingly important due to smaller batch sizes in personalized medicine.
Eliminate sources of error
Problems that need to be investigated during production or filling and finishing are common. The ability to trace back certain machinery events or previously reported issues speeds up root cause analysis and reduces the number of blocked products due to ongoing investigations.
Increase efficiency
Smaller batch sizes are also making work processes more complex and flexible. Line changes can be simplified and accelerated if the batch affiliation, filling status or other product information can be traced for each individual product thanks to coding.
Limit damage in case of recalls
In the event of a drug recall, the link between the unique code of the primary packaging and the serialized medicine makes it possible to draw quick and precise conclusion about the affected product units.
Fight counterfeiting
A unique code on the primary packaging that cannot be removed, along with the corresponding digital identity, is another important step on the way to a counterfeit-proof product.
Enable patient and end-customer interaction
Unique codes or NFC technology enable a variety of use cases in marketing, customer profiling, or patient safety, allowing end customers and patients to communicate directly with the product via smartphone.
Support circular economy
The trend toward reusable packaging materials goes beyond the cosmetics industry. Clearly identifiable products and their digital tracing enable the continuous tracking of products in the market.
Compliance
By means of direct marking packaging can help improve product and process quality and its documentation, making it possible to address the increasingly stringent regulatory requirements.
Gerresheimer is a leading global partner to the pharma and healthcare industry. With specialty products made of glass and plastic, the company contributes to health and well-being. Gerresheimer is represented worldwide and produces with around 10,000 employees wherever its customers and markets are. With plants in Europe, North and South America and Asia, Gerresheimer generates sales of around €1.5 billion. Its wide range of products includes pharmaceutical packaging and products for the simple and safe administration of medicines: Insulin pens, inhalers, micropumps, prefillable syringes, injection vials, ampoules, bottles and containers for liquid and solid medications with closure and safety systems as well as packaging for the cosmetics industry.
Gerresheimer AG
Klaus-Bungert-Straße 4
40468 Düsseldorf
Telefon: +49 (211) 6181-00
Telefax: +49 (211) 6181-295
http://www.gerresheimer.de
Senior Manager Group Communication
Telefon: +49 (211) 6181-246
Fax: +49 (211) 6181-241
E-Mail: m.stolzenwald@gerresheimer.com
Group Senior Director Marketing & Communication
Telefon: +49 (211) 6181-250
E-Mail: ueli.utzinger@gerresheimer.com
![]()