- More than ten million people worldwide suffer from decompensated cirrhosis, often as a result of severe alcoholic hepatitis (sAH) or other chronic liver diseases.
- In its final stages, decompensated cirrhosis leads to acute-on-chronic liver failure (ACLF), a syndrome characterised by multi-organ failure. Effective treatment of ACLF is an urgent and unmet need.
- The A-TANGO Consortium will perform Phase II clinical studies of an innovative therapeutic strategy that targets inflammation and improves liver regeneration. We call this novel combinatorial therapy “G-TAK”.
- In addition, A-TANGO strives to identify reliable biomarkers for better patient stratification and selection for novel therapies.
Why are novel therapies for ACLF urgently needed?
Liver diseases that lead to cirrhosis, in other words scarring of the liver, include autoimmune disorders, fatty liver, as well as bacterial or viral infections. Alcohol abuse, however, accounts for the majority (30-40%) of all liver-related deaths in Europe. A severe form of alcoholic liver disease is called alcoholic hepatitis (sAH), which carries high mortality rates. Regardless of the cause, when cirrhosis progresses too far, the body can no longer compensate for the dysfunctional liver. This results in fluid accumulation in the abdomen (ascites), impaired brain function (hepatic encephalopathy), and bleeding (gastrointestinal haemorrhage). Untreated, decompensated cirrhosis eventually leads to acute-on-chronic liver failure (ACLF) and death. Aside from enormous individual suffering, ACLF has a major socio-economic impact because of high health care costs and the patients’ inability to work or seek employment. Currently, an alarming 30-40% of the patients that suffer from ACLF die within three months, despite best available medical treatment. Treatment options are still limited, leaving liver transplantation as the last resort. Thus, effective treatment approaches are urgently needed in order to decrease the mortality rate of patients with ACLF. Research has demonstrated that ACLF is caused by inflammation and the inability of the liver to regenerate.
G-TAK is an innovative, novel treatment strategy that combines the strengths of two biologically active ingredients and provides new hope for patients. Preclinical studies in rodent models of liver failure indicate an extremely high treatment efficacy for G-TAK, while clinical studies to determine the dosage, safety, tolerability, pharmacokinetics (how the body processes a drug), and pharmacodynamics (how a drug affects the body) of each of the two ingredients by itself have already been completed. A-TANGO now takes it to the next level by testing this novel combinatorial treatment, G-TAK, in a large, randomised, controlled Phase II clinical trial to determine its efficacy in sAH- and ACLF-patients across Europe.
How will A-TANGO help ACLF-patients and advance research?
The A-TANGO project started on 1 March 2021, and received research funding from the European Commission for five years. A-TANGO aims to provide proof of concept data of G-TAK in ACLF patients that would allow initiation of late-phase clinical trials to improve the survival rate, treatment outcome, and quality of life for sAH- and ACLF-patients. Of course, A-TANGO will also expand the scientific understanding of the underlying pathophysiological mechanisms that lead to decompensated cirrhosis, sAH, and ACLF. The consortium also aims to identify biomarkers for better patient stratification, which may improve overall treatment outcome.
If G-TAK proves to be efficacious, conservative predictions indicate that A-TANGO has the potential to increase the survival rate of ACLF-patients by 20%. This would imply approximately 12,000 fewer patients on the waiting list for a liver transplant in Europe per year, about EUR 60,000 savings per patient, and up to EUR 720 million savings with regard to healthcare costs in Europe each year. Another key goal of A-TANGO is increasing societal awareness, reducing stigmatisation and deepening scientific literacy regarding end-stage liver disease.
Who constitutes the A-TANGO research consortium?
The project’s scientific coordinator, Prof. Rajiv Jalan (MD, PhD), is the scientific director of the European Foundation for the Study of Chronic Liver Failure (EFCLIF) and the head of the liver failure group at University College London (UCL). Hepatologist Dr med. Cornelius Engelmann (Charité) spearheads the clinical trial as principal investigator, and concentris research management gmbh manages the project and communicates its progress to the public. The research consortium consists of 14 institutions, including five university hospitals, six small and medium-sized enterprises (SMEs), including Hepyx Ltd. (which owns background intellectual property), two liver research associations, and the European Liver Patients Association (ELPA). Profs Rajiv Jalan, Cornelius Engelmann, and Dr Fausto Andreola together with the help of other colleagues from UCL invented the novel combinatorial therapy, G-TAK, which will soon be implemented at 30 clinical sites across Europe.
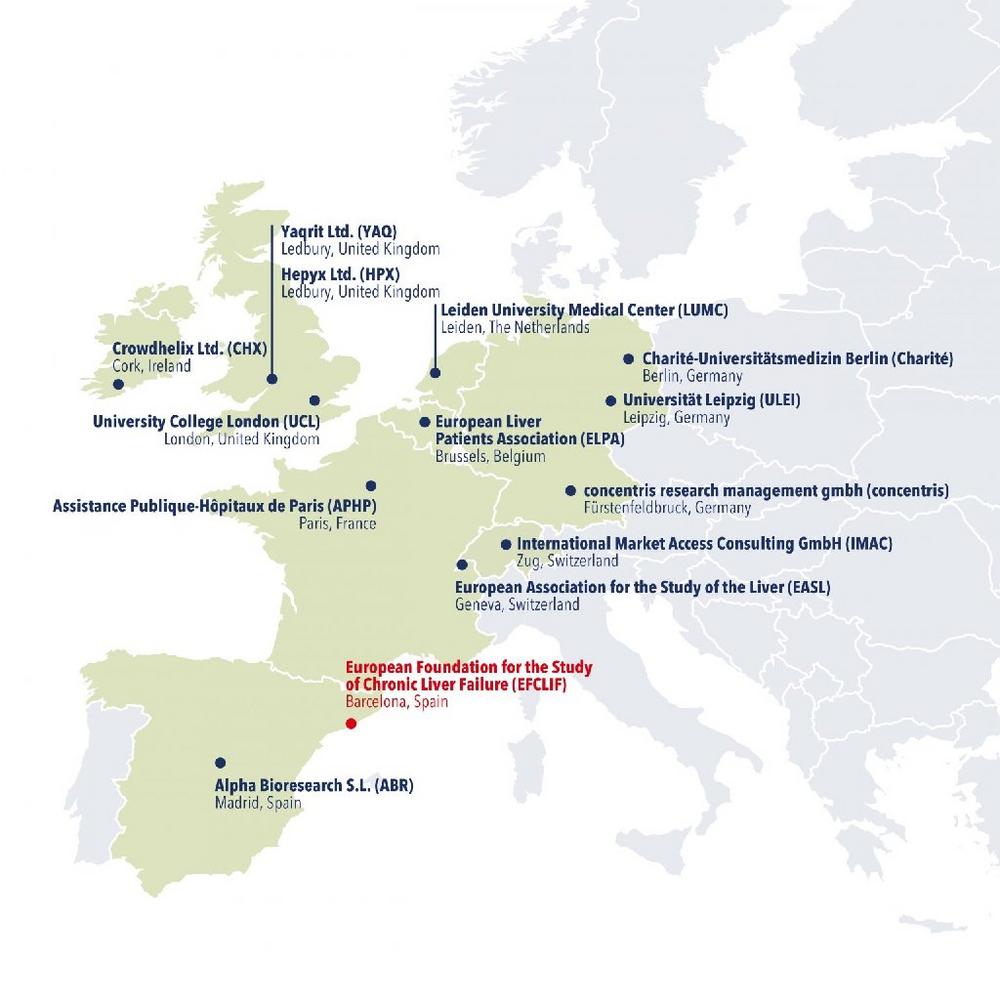
These are the members of the A-TANGO consortium:
- Alpha Bioesearch S.L. (ABR), Spain
- Assistance Publique Hôpitaux de Paris (APHP), France
- Charité – Universitätsmedizin Berlin (Charité), Germany
- concentris research management gmbh (concentris), Germany
- Crowdhelix Ltd. (CHX), Ireland
- European Association for the Study of the Liver (EASL), Switzerland
- European Foundation for the Study of Chronic Liver Failure (EFCLIF), Spain
- European Liver Patients Association (ELPA), Belgium
- Hepyx Ltd. (HPX), United Kingdom
- International Market Access Consulting GmbH (IMAC), Switzerland
- Leiden University Medical Center (LUMC), The Netherlands
- Universität Leipzig (ULEI), Germany
- University College London (UCL), United Kingdom
- Yaqrit Ltd. (YAQ), United Kingdom
About EASL and the A-TANGO Consortium
The A-TANGO Consortium, a five-year project, consists of 14 institutions across eight countries in Europe. It aims to perform Phase II clinical studies of an innovative therapeutic strategy that targets inflammation and improves liver regeneration – a novel combinatorial therapy “G-TAK”. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 945096.
The 14 institutions of the consortium include five university hospitals, six small and medium-sized enterprises, two liver research associations including EASL, and the European Liver Patients Association (ELPA).
EASL serves as the dissemination arm of several EU-funded projects, including A-TANGO. Accordingly, we will provide support to A-TANGO, in providing dissemination to our established communication channels to reach the wider liver community.
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 945096. This press release reflects only the view of the authors, and the European Commission is not responsible for any use that may be made of the information it contains.
EASL
7 rue Daubin
CH1203 Geneva
Telefon: +41 (22) 8070360
http://easl.eu
A-TANGO Coordinator
E-Mail: rajiv.jalan@efclif.com
Principal Investigator
E-Mail: cornelius.engelmann@charite.de
Dissemination Manager
E-Mail: nina.donner@concentris.de
![]()